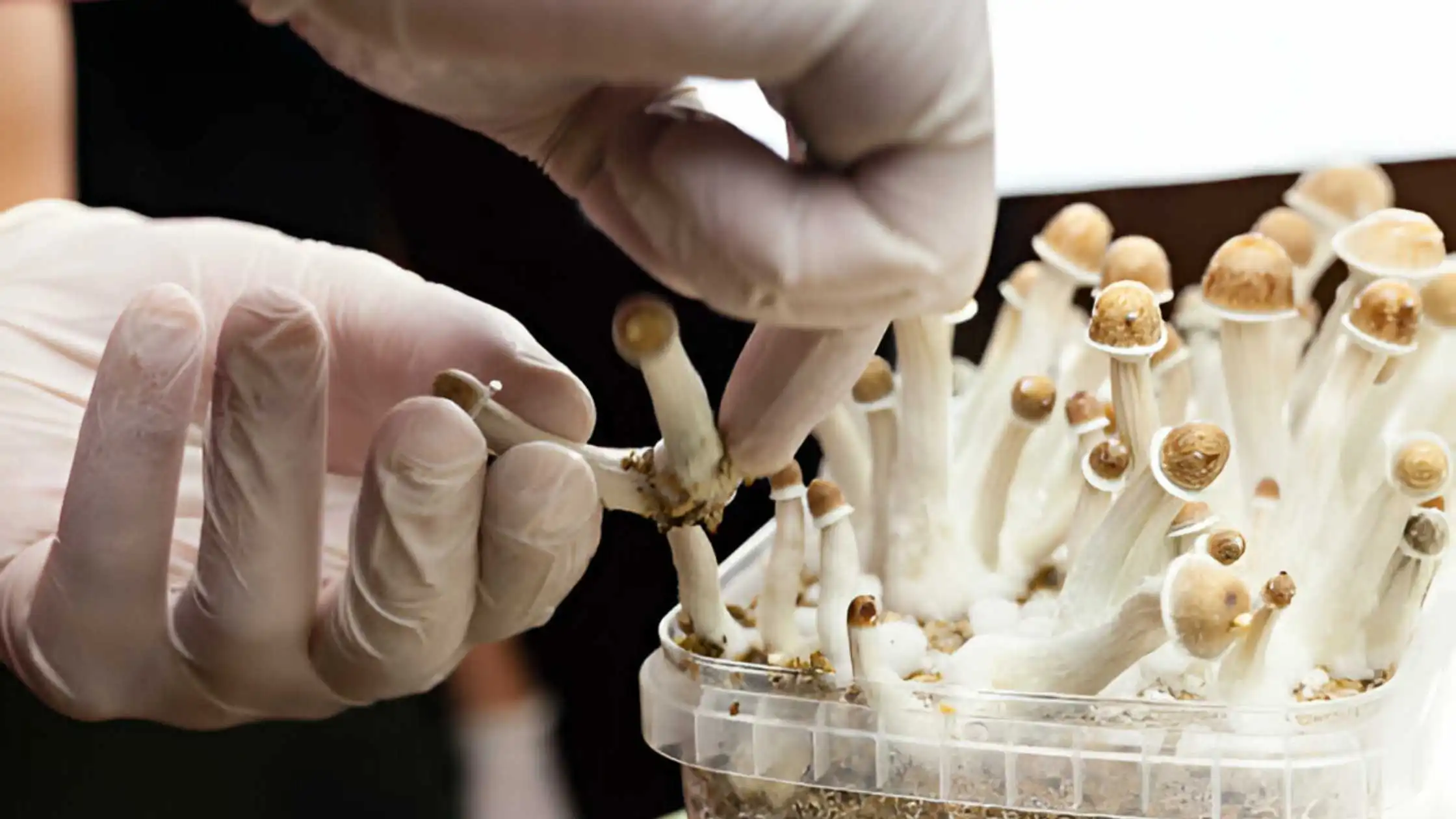
A recently filed lawsuit raises questions about current psilocybin law in Canada.
Is legalized psilocybin in Canada coming soon? Will it follow the same legal pathway as cannabis? Can it be constitutionally sound?
A recently filed lawsuit on behalf of seven patients and one caregiver raises these questions about the current psilocybin law in Canada, which could change the government-as-gatekeeper model that currently exists.
“Constitutionally viable access requires a doctor-as-gatekeeper model,” the lawsuit stated.
No trial date has been set yet, but a case management conference is expected in late October. The outcome of the lawsuit is being closely watched by psilocybin business developers around the world – especially in the U.S.
Current situation
According to Health Canada, as psychoactive substances, psilocybin and psilocin are controlled internationally under the United Nations Drug Control Conventions and, in Canada, under Schedule III of the Controlled Drugs and Substances Act (CDSA).
Under the CDSA, activities with magic mushrooms, psilocybin and psilocin, such as sale, possession, and production, are illegal unless explicitly authorized by Health Canada. This can happen through the issuance of a license or exemption, for example.
And like all drugs, magic mushrooms, psilocybin, and psilocin also are subject to the Food and Drugs Act.
The Canadian government points out that psilocybin can legally be accessed through clinical trials and Health Canada’s Special Access Program (SAP), and people should pursue these paths unless it can be demonstrated that access to psilocybin is not possible or suitable through those existing legal routes.
According to Health Canada, both clinical trials and the SAP have safeguards and requirements in place to protect the health and safety of patients, help ensure the quality of the drug, and provide for administration and oversight by a qualified professional.
“The Special Access Program and individual exemptions from the CDSA are not mechanisms to encourage the early use of unauthorized drugs, nor are they meant to be used as a means of circumventing clinical development or the established drug review and approval process,” the agency said.
If a qualifying patient wants to get psilocybin, they can get it from individuals, certain organizations that follow CDSA guidelines, or by foraging for psilocybin mushrooms in the wild, the lawsuit points out, adding that “an individual lacking training in mycology attempting to forage for natural psilocybin mushrooms in the wild could inadvertently poison themselves.”
Before the July lawsuit, in February, a charter challenge to Health Canada over psilocybin access for healthcare practitioners in response to the government’s denials of exemptions was submitted by the nonprofit advocacy group Therapsil, which is leading an effort to make psilocybin-assisted therapy available to Canadian patients.
Therapsil has been a key advocacy source to help get exemptions for patients and healthcare providers. The organization has supported 55 patients in five different provinces to access legal, psilocybin-assisted psychotherapy as of the beginning of this year.
As of February, Therapsil had a waitlist of more than 800 patients requesting assistance, including 13 patients who submitted affidavits for the challenge. All of them were on that list because of a lack of qualified psilocybin therapy healthcare practitioners.
The Therapsil challenge to Health Canada included written representations from practitioners about the need for exemptions for them to “possess, transport, consume, and destroy psilocybin” as part of an experiential training program in psilocybin-assisted psychotherapy.
“The enormous harm that will be caused by refusing these exemptions far outweighs any negligible benefit that refusing these exemptions might confer,” the challenge stated.
Cannabis as precedent
The challenge also noted a precedent to change how the government could work with psilocybin by citing two landmark cannabis cases, where Canadian courts ruled that the absolute prohibition of cannabis by the CDSA is wrong: R v. Parker in 2000 and R. v. Smith 2015 in the Supreme Court of Canada.
In R. v. Parker, Terry Parker was charged in 1996 after a police raid on his home in which the cannabis plants he was growing to control his epileptic seizures were confiscated. The court held that the prohibition on the cultivation and possession of marijuana was unconstitutional.
In R. v Smith, Owen Edward Smith was charged with possession of cannabis and making and selling edibles and other cannabis extracts. In an appeal confirming Smith’s acquittal, the judges concluded that “the prohibition of non-dried forms of medical marihuana limits liberty and security of the person in a manner that is arbitrary and hence is not in accord with the principles of fundamental justice.”
“These rulings compelled the federal government to amend the CDSA to allow for the medical use of cannabis,” Therapsil’s challenge stated. “The decision to use cannabis medically is now made between patient and doctor, with no requirement for bureaucratic approval.”
The challenge argues that the pathways to treatment as outlined by Health Canada – obtaining a personal exemption from the Minister of Health under subsection 56(1) of the CDSA, working with a doctor to obtain authorization through Canada’s SAP, or enrolling in a clinical trial—do not adequately serve the needs of patients.
“A clinical trial would be unethical,” the challenge argues. “There is no genuine uncertainty in the expert community about the safety of psilocybin for healthy adults in a training program. Best practices for experiential training are also already well documented. It is unethical to conduct a clinical trial on questions that have already been answered because this imposes an unnecessary burden on participants. It is unethical to conduct a trial when there is no genuine uncertainty in the expert community about the research question at issue.”
The psilocybin access issue may already be moot in Canada. One of the plaintiffs in the lawsuit, Thomas Hartle, was originally granted a one-year exemption for psilocybin use, and on August 12, 2020, with the help of Therapsil, he became the first Canadian to legally use psilocybin-assisted psychotherapy to treat the anxiety connected with his stage 4 colon cancer diagnosis. But his application for another year’s exemption is in limbo.
There is a growing belief that whatever Canada decides will have a direct impact on the U.S. and this country’s psilocybin legalization efforts.
“If the lawsuit prevails, it’s going to be a revolution,” Marc Goldgrub, a Toronto-based lawyer with Green Economy Law who specializes in psychedelics, told Bloomberg. Narrow medical use may pressure Canada to allow more widespread use for wellness, similar to Oregon’s approach, he said.
“Money talks. I think if they open an individualized medical program, so much money is going to come to Canada. And that money will push for a lot more,” Goldgrub said.
From there, the pressure would be on the U.S. to move forward with its versions of legalization to stem an outflow of cash and talent to Canada, he predicted.
“We’re already seeing that money here,” he said. “I’m already getting calls from Americans who want to get involved.”
Disclaimer: https://www.greenmarketreport.com/is-broader-access-to-psilocybin-in-canada-on-the-horizon/
Posted by: Times Of Hemp, TOH, #TOH, #TimesOfHemp, https://www.timesofhemp.com